Catalysis plays a major role in today’s society, impacting an estimated 90% of all commercially produced chemical products. Generating about a trillion USD in products worldwide, catalysis is used in energy processing (petroleum refining, steam reforming, automotive catalytic conversion, fuel cells), bulk and fine chemical manufacturing, food processing, and environmental protection.
Catalysis is a process in which an added catalyst enhances the rate of reaction by lowering its reaction energy threshold. Most catalytic processes employ heterogeneous catalysis, where the catalyst is solid whereas the reactant fuel is in the liquid phase. Carbon plays a diverse role in heterogeneous catalysis, most prominently featured as a solid support for metal catalysts, for example on fuel cell electrodes. In some processes, carbon itself acts as a catalyst.
Graphene, as a form of carbon with a high electron mobility and specific surface area, arises as a natural candidate as a catalytic support. In the most common catalytic reaction, a noble metal catalyst is anchored to a support that acts not only as mechanical support, but also provides charge transfer that assists the reaction. In its purest form, graphene is a chemically inert surface, because there are no free chemical bonds. However, the addition of strain, defects, or functional groups radically changes the chemical properties of graphene and makes it suitable as catalytic support.
A desirable method for the immobilization of metal complexes is the formation of a covalent bond between the solid support and a ligand binder. In the case of graphene this general method has two important drawbacks. First arising from the need of an additional functionalization of the metal complex and the graphene solid surface (which, in turn implies an increase in the catalyst preparation cost) and second, arising from the possible changes in chemical reactivity and therefore in catalytic activity, derived from the formation of the covalent bond.
As an alternative, non-covalent interactions are emerging as a powerful tool that gives rise to supported catalysts without chemical modification of the homogeneous catalyst or the graphene. The most common non-covalent interactions are hydrogen bonding, van der Waals forces and π-stacking. The relative energy of a single non-covalent interaction is much lower than a typical covalent bond but on account of their multiplicity, the overall influence should not be negligible on the anchoring of the metal complex on the surface of graphene.
The main advantage of immobilizating a homogeneous catalyst by non-covalent interactions is the simplicity of catalyst design and availability. Further modifications to achieve high selectivity and stability are easily obtained by tuning the electronic and steric properties. The well defined supported molecular complexes for catalytic applications should facilitate precise characterization of the hybrid materials and the study of the reaction mechanisms. The reaction mechanism could be studied in solution at the molecular level with the well-defined homogeneous organometallic techniques. The support may play a role during the catalytic process, not acting as a simple platform for anchoring the molecular complex, but also enhancing the catalytic activity by avoiding aggregation and facilitating the interaction between the substrates and the catalytic active species.
Non-covalent interactions by means of π-stacking are a particularly attractive method for immobilization of metal complexes onto graphene, because the number of functional groups in this material is limited. Moreover, non-covalent interactions between the catalyst and the support are arising as promising alternatives to the more widely used covalent interactions because they also avoid the functionalization of both, the catalyst and the surface, which may turn in the modification of the inherent properties of the catalyst and the graphene.
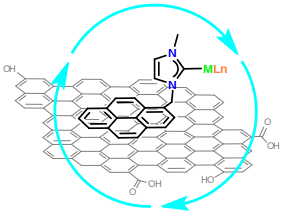
Image: Graphene as catalytic support (courtesy of Prof. Jose A. Mata Martínez, Departamento de Química Inorgánica y Orgánica, Universitat Jaume I, Castellon)
Metal complexes of Pd and Ru have been anchored on the surface of graphene by non covalent interactions under mild reaction conditions. The results reveal that the metal complexes are strongly anchored on the surface of graphene. The catalytic properties of the hybrid material reveal that the catalytic activity is increased when the metal complex is immobilized on the surface of graphene. The system is stable and can be reused without any decrease in activity.
To conclude, graphene is an excellent candidate for use as catalytic support, especially in the emerging technology of non-covalently bonded supports, which offer simpler fuel cells at a reduced cost compared to current technology. The most useful form of graphene for catalysis so far is reduced graphene oxide, rGO, which is one of our most popular products. This form of graphene is low-cost and easily integrated into matrices.
via Graphenea
No comments:
Post a Comment